Human apolipoprotein B protocol update
Published: December 13, 2024
Updated: February 13, 2025
2 minute read
Authored by: Lara Mentlein
Update December 13, 2024
We have updated the datasheet of our ELISA Pro: Human apoB kit.
The update concerns our guidelines for the dilution of serum and plasma samples. To be specific, an initial dilution of serum/plasma in Apo ELISA buffer is now recommended. The Sample solvent treatment (previously the first step of the protocol) now follows as a second step (Figure 1).
| New | Previous guidelines |
|
Image
|
Image
|
Figure 1: Comparison of our new to our previous guidelines for the dilution of serum and plasma samples when using our ELISA Pro: Human apoB kit.
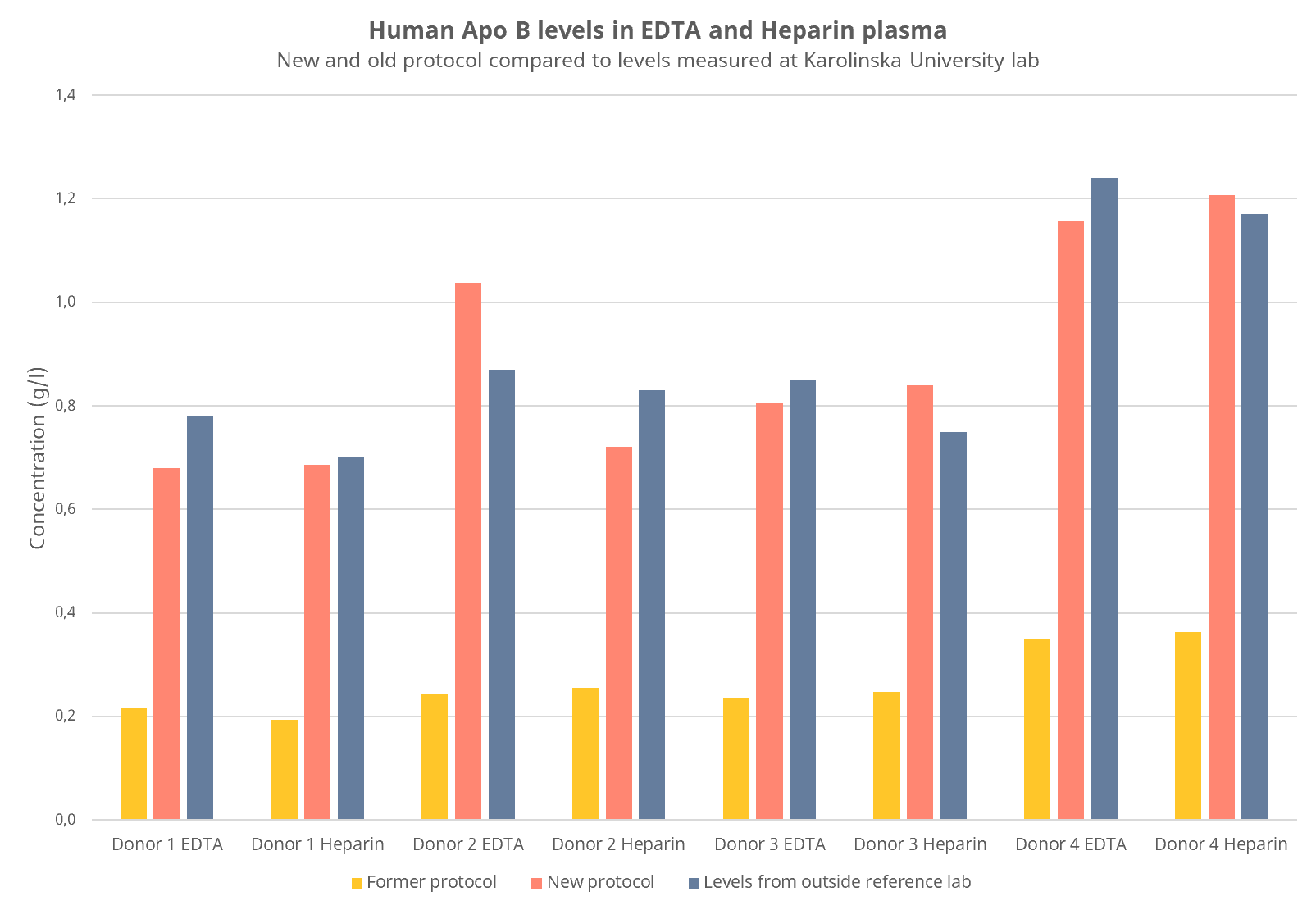
In our hands, the updated protocol yields higher values, but closer to the results from an outside reference lab (Figure 2). Keep in mind that results are higher with the new protocol than with the old, if you plan to compare results to a previous experiment. By following this updated protocol, we can ensure that your results will become reliable and more accurate.
You can find the updated datasheet including the new protocol on the product page of the ApoB kit:
Don’t hesitate to contact us with any questions regarding this protocol update.
Figure 2: ApoB concentration was measured in plasma from blood collection tubes with different additives (EDTA or Heparin). Results with our new and previous guidelines were compared to results obtained from an outside reference lab (Karolinska University Hospital lab) for four blood donors.
Update February 13, 2025
We have renamed one of the buffers included in our ELISA Pro: Human apoB kit.
We have renamed our Triton-X 100 buffer to Sample solvent. The composition of the buffer remains the same. This buffer is one of the components of our ELISA Pro: Human apoB kit.