Background spots in negative controls of ELISpot and FluoroSpot
Published: February 19, 2024
Updated: December 20, 2024
5 minute read
Authored by: Tyler Sandberg
The ELISpot and FluoroSpot assays are extremely sensitive functional assays that can detect even the rarest antigen-specific cells. When setting up your experiment, necessary controls are essential for success, but they don’t always turn out as expected (and that's frustrating). Let’s learn why spots might appear in your negative or background control wells in the assays and how to interpret these results correctly.
If you are consistently experiencing high background spots in ELISpot and FluoroSpot, it's good to revisit the assay controls. Reliable assay controls are essential for any experiment to generate trustworthy and accurate data. We recommend including the following three control conditions in each ELISpot or FluoroSpot experiment:
-
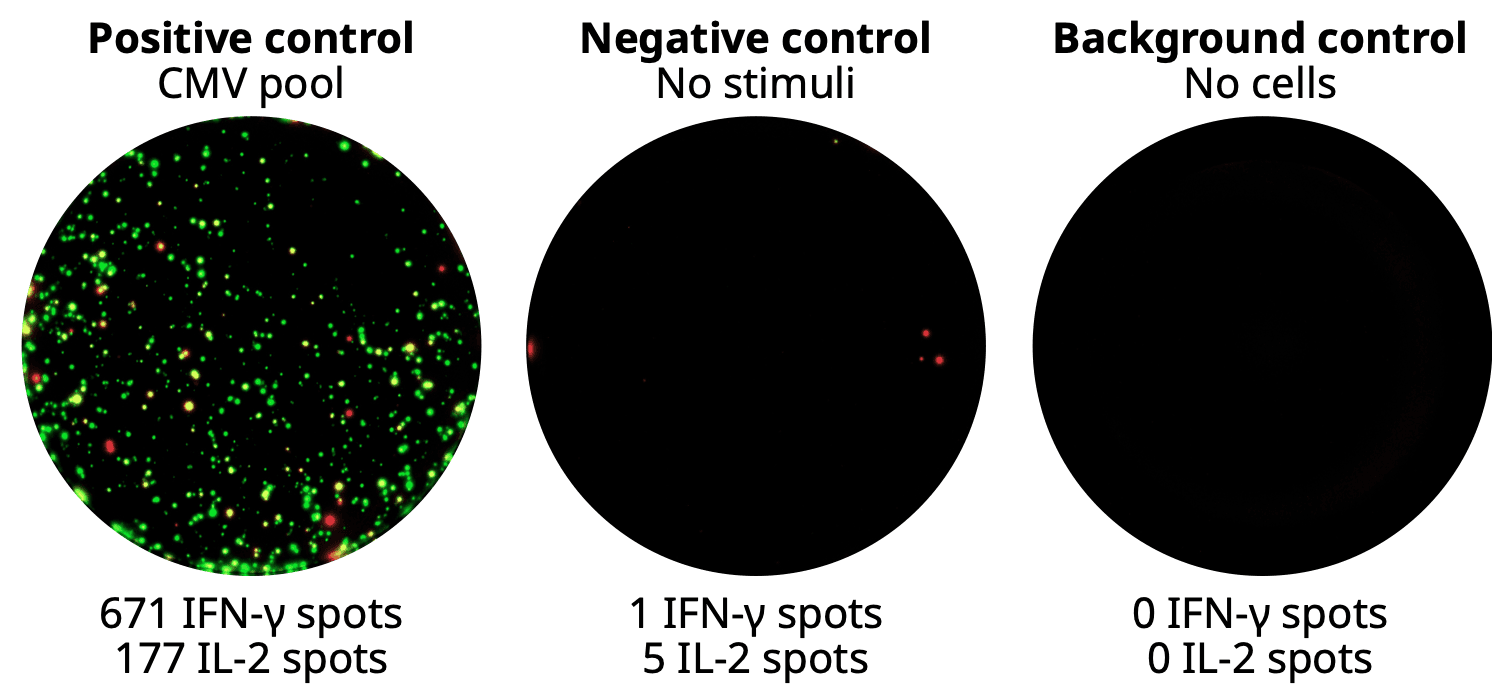
Positive control: validates assay functionality and cell reactivity, typically using a polyclonal stimulator or common antigens like a CEF, CMV, or EBV pool.
-
Negative control: contains cells without stimuli, reflecting the baseline of spontaneous secretion. It should match the medium and DMSO levels used elsewhere.
-
Background control: includes all reagents except for the cells to check for non-specific spot formation caused by reagent aggregates or particulate contaminates in buffers.
Control well images from an ELISpot Plus: Human IFN-γ (ALP) kit.
Control wells from a FluoroSpot Plus: Human IFN-γ/IL-2 kit (IFN-γ in green, IL-2 in red).
Using these controls alongside your antigen-stimulation experimental conditions gives you the best chances for success in your ELISpot or FluoroSpot assays. You can read more about these controls and proper assay execution in our step-by-step guides for ELISpot and FluoroSpot.
One can hope for a “perfect” assay with an even distribution of small, well-defined spots, no artifacts, and very low or no background spots in the negative control wells, but despite meticulous planning, background spots in ELISpot and FluoroSpot can still occur. The European ELISpot Proficiency Panel noted that a “typical” background level is below 6 spots per 100,000 PBMCs for IFN-γ ELISpot (Moodie et al., 2010). Make sure to read the entire paper to fully grasp how to interpret your background responses.
Variability can arise from the donors' health over time or infection status, but spots in negative controls don't necessarily indicate a flawed experiment. Comparison with your antigen-stimulated conditions is crucial for interpretation. When determining a positive response, we recommend the distribution-free resampling (DFR) statistical test. Learn more in our detailed blog post on the subject.
Besides spontaneously secreting cells, spots can also show up in the negative controls when something is off in the experimental setup leading to non-specific stimulation of immune cells. This can occur due to various factors in the culture conditions that inadvertently stimulate the cells, leading to the production of the analyte of interest. Several factors could be at play resulting in an increased background signal in the negative control wells:
-
Contaminants in reagents: endotoxins or other contaminants can be present in the medium, serum, or DMSO that activates the cells in the sample.
-
Always test new batches of serum to ensure optimal experimental conditions.
-
-
Cell handling: immune cells can be sensitive to physical stress from temperature fluctuations and other handling procedures.
-
Proper cell handling and resting are important when working with functional assays.
-
Download our cell handling guide to learn how we do things at Mabtech.
-
-
Overcrowding of cells: cell-to-cell contact is important in ELISpot, but overcrowding could lead to unwanted nonspecific activation of cells in a sample.
-
A good starting point for cell numbers is between 200,000-300,000 cells for antigen-specific wells
-
50,000 cells can be suitable for positive control wells.
-
-
Insufficient washing steps: cells and unbound reagents need to be properly washed away. Remaining cells or reagents can lead to an unwanted high background.
-
Follow protocols for the proper number of washes for both manual and automated washing setups.
-
-
Artifacts: dust particles and reagent aggregates can lead to high background in negative controls. Working in proper lab conditions and filtering reagents according to the protocol can reduce the number of artifacts present in control wells.
Essential controls and optimizing assay conditions, including cell density, incubation times, and reagent quality, can help you assess and minimize the impact of nonspecific stimulation on the assay's background. We hope this post has brought some light onto why it can be ok to have a “normal” amount of background spots in negative controls as well as how to further optimize conditions when high background spots occur in ELISpot and FluoroSpot. For more tips and guides, check out our Knowledge Hub or the excellent book, ELISpot for Rookies (and Experts Too) by Sylvia Janetzki.
Note: Maintaining rigorous cleanliness standards in animal facilities is crucial for minimizing the risk of spontaneous in vivo activation of immune cells, which can significantly impact the reliability and reproducibility of ELISpot. Spontaneous activation can result from exposure to pathogens, environmental stressors, or contaminants, leading to an altered immune status and baseline activity in the animals. By ensuring a controlled, clean environment, you can reduce the incidence of unintended immune responses, ensuring that cell responses observed in assays are primarily due to experimental conditions rather than environmental factors.
References